Updated January 2017
The use of systemic and or radiation therapy prior to surgery (neoadjuvant therapy or NAT) has been studied since the early 1970s. There are several potential advantages to the use of NAT1:
- Allows for immediate assessment of tumour response
- Allows for evaluation of new and novel agents
- Allows for evaluation of change in biomarkers with treatment
- May allow for earlier control of micro-metastases
The key landmark study by Wolmark et al in 2001 (NSABP B-18) demonstrated that NAT (with chemotherapy) was safe, with no difference in outcomes of disease free survival (DFS) or overall survival (OS) in patients who received either pre-operative chemotherapy or post-operative chemotherapy2. It was demonstrated, however, that patients who had a complete pathological response (pCR) to NAT had an improved OS compared to those who did not achieve a pCR with NAT.
NAT has been since studied in multiple settings, tumour subtypes, and with many different types of agents. NAT may be considered in certain patients with early stage breast cancer, and is the standard of care for patients with locally advanced breast cancer or inflammatory breast cancer.
The neoadjuvant treatment of breast cancer requires a coordinated effort amongst surgical oncology, medical oncology, radiation oncology, nursing, pathology, radiology and clerical booking staff.3
- Patients with locally advanced breast cancer (LABC)3,4,5,6,7
- LABC is generally accepted as T3 or T4 tumours of any clinical N status or any tumour size with clinical N2 or N3 disease, operable or inoperable. Inflammatory breast cancer is included within this definition.
- Patients with early stage breast cancer (≥2cm) and chemo-responsive tumour markers (i.e. triple negative, Her2+), who will benefit from downsizing for breast conserving surgery (BCS)6
- Absolute or Relative contraindications to surgery (advanced age/multiple medical comorbidities) in the setting of estrogen receptor positive tumours (for consideration of neoadjuvant endocrine therapy)3
In general, any patient who is a candidate for adjuvant systemic therapy can be considered for neoadjuvant chemotherapy. Patients with tumours < 2cm, low grade, ER positive/Her2 negative, may not require systemic chemotherapy and should undergo surgery first.8
Referral process
- If a patient is felt to be an appropriate candidate for NAT by a surgeon or family physician, an urgent referral should be sent to BC Cancer indicating a consultation request for NAT.
- The patient should be seen before or at the beginning of NAT by a surgeon to determine the tentative surgical plan and to ensure all essential biopsies and imaging (e.g. axillary), and clip placement have been organized and/or completed.
- A radiation oncologist should be consulted early in the patient's course, as the tumour can change dramatically in size and volume from initial presentation due to NAT.
Work up
Before starting NAT the following tests and procedures should be completed:4,6,7
- Biomarker status (estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (Her2) on core biopsy
- Bilateral mammogram +/- breast ultrasound
- Accurate tumour measurement on clinical exam - consider breast MRI before initiation of NAT to assess tumour extent, especially if patient may be a BCS candidate
- Clip should be placed in tumour if patient is a candidate for, or may be considering, BCS
- Imaging of chest/abdomen plus bone scan to assess for distant metastases
- Suspicious lymph nodes on exam or imaging should undergo fine needle aspiration prior to initiation of NAT
How should patients be followed during NAT?
- Patients should be assessed at each cycle of chemotherapy for clinical response with accurate clinical measurement.
- There are no standard recommendations for assessing radiologic response. MRI may be considered to assess radiologic response and to aid surgical planning.3,9
- Patients should be seen by their surgeon halfway through NAT and prior to their last cycle of chemotherapy to determine final surgical plan. In addition, a more urgent assessment should be completed if the tumour is not responding to NAT.
- Patients on neoadjuvant endocrine therapy can be assessed monthly.3
Systemic Therapy
- Options for systemic therapy are outlined in the section below. Cancers that are responding should continue the planned treatment protocol.
- Hormone therapy should be given after completion of NAT if cancer is ER positive (See "Early Invasive Breast Cancer".
- If the cancer is her2 positive, trastuzumab should be given for one year, starting during the non-anthracycline portion of the chemotherapy.
Locoregional Therapy
Following NAT, patients should undergo definitive locoregional therapy, which usually involves surgery and radiation.
Surgery should be planned for 3-4 weeks following completion of neoadjuvant chemotherapy to allow adequate immune and general recovery first. This is consistent with timelines reported in randomized controlled trials of NAT in breast cancer patients.
Surgical considerations - Breast
- Breast-conserving surgery can be considered for appropriate patients
- Mastectomy should be considered for patients with contraindications to radiation therapy, multicentric disease, incomplete response of skin involvement, and large tumour to breast size ratio after NAT and potential for poor cosmesis.3.4.7
- Mastectomy with axillary lymph node dissection is the recommended surgery for patients with inflammatory breast cancer.
Surgical considerations - Axilla
- Pre-NAT N0:
- Sentinel lymph node biopsy after NAT at the time of definitive surgical management should be completed3,6,9
- Pre-NAT N+:
- ALND is currently recommended.
- SLNB after NAT remains controversial in this patient population. Ongoing studies continue to investigate SLNB after NAT in node positive patients and therefore, related literature should be reviewed and this practice re-considered in future updates9
Radiation Therapy
- In patients with resectable disease, adjuvant locoregional radiotherapy typically starts approximately 6 weeks after surgery to allow for proper healing for patients with node positive disease
- In patients with disease that is still unresectable after completion of neoadjuvant chemotherapy, or who have disease that progresses on chemotherapy, locoregional radiotherapy is delivered earlier in the course of treatment
- Radiation therapy for N0 tumours should be routinely recommended after BCS and for select patients after mastectomy
- See "Radiation Therapy" section for details of radiotherapy
Reconstruction
- If a mastectomy with autologous tissue reconstruction is planned, radiation is generally preferred prior to surgery, to minimize the negative cosmetic effect on the reconstructed breast of radiation. In other circumstances, radiation generally follows surgery. Timing of radiation should be coordinated between radiation oncology and the surgical team.
Progression or remains inoperable on NAT
Patients with technically inoperable tumours after NAT should have radiotherapy after chemotherapy with a view to further local downstaging. If their disease becomes operable, appropriate surgery should follow.
If the tumour enlarges while on NAT, treatment should be changed. Non-cross resistant chemotherapeutic agents, salvage radiotherapy, or surgery are all appropriate options to consider in this setting. Discussion of these cases at a multi-disciplinary tumour board should be considered. Cancers showing equivocal response may benefit the most from tumour board multidisciplinary review, to explore ways to improve clinical response.
Pathology findings following NAT are designated with a 'y' in front of the 'p" (example ypT2N1) to reflect that the cancer has been exposed to treatment. In general, the less disease present, the better the prognosis, with a large jump (improvement) in prognosis occurring with a pathological complete response (pCR).10 The improved disease free and overall survival associated with a pCR has been shown in cancers of all biomarker profiles (triple negative, ER positive, and her2 positive).11
The definition of pathological complete response varies in clinical trials. The strictest definition allows no residual invasive disease or in situ disease in the axilla or breast. The least strict definition considers only the absence of invasive disease in the breast, allowing for residual nodal metastases. The Canadian expert consensus for the definition of pathological complete response is no evidence of invasive disease in the breast or axilla3. This definition allows for residual DCIS and is consistent with the definition most often reported in clinical trials of NAT.
There is not data to support that additional chemotherapy is of benefit in patients who have residual disease at surgery following NAT. Clinical trial options can be considered if available. Hormone therapy and trastuzumab should be given according to standard of care.
Chemotherapy
Preoperative chemotherapy (NAT) has several functions:
- to control/ eradicate micrometastases,
- to improve the loco-regional control
- to render the patient operable, if they presented with inoperable disease.
In general, there is a preference for chemotherapy regimens that include an anthracycline and a taxane, either concurrently or sequentially
Chemotherapy Breast Protocols, based on demonstration of improved outcome compared with anthracycline only regimens.
14 Recommended regimens include BRLAACD and UBRAJACTW, but avoidance of anthracyclines or taxanes, or abbreviated courses, may be necessary for some patients with co-morbidities. Trastuzumab (BRLAACDT) should be added if the cancer is her2+. NAT is typically given for about six months (8 cycles) prior to definitive local therapy, provided there is no adverse progression of the disease on therapy.
Hormone Therapy
The likelihood of achieving a pCR with endocrine therapy is significantly lower than with chemotherapy.
Neoadjuvant endocrine therapy may be an appropriate option in patients found to have hormone receptor positive disease and who are unfit for chemotherapy. Canadian experts from the Canadian Consensus for the Treatment of Locally Advanced Breast Cancer recommend endocrine therapy for patients with ER+PR+Her2- disease and multiple co-morbidities, or who are older than 80 years. The duration of neoadjuvant hormone therapy is not well defined, but studies have been conducted using an interval of 4-8 months.12 The overall goals of care should be discussed with the patient and the duration of therapy should reflect this (ie if the goal is curative then a pre-defined duration of NAT with endocrine therapy followed by surgery; if the goal of care is control then there may be no pre-defined duration of NAT).
Aromatase inhibitors and Tamoxifen have been studied in the neoadjuvant setting.13 In serial biopsy studies, aromatase inhibitors have demonstrated a greater drop in proliferation markers (Ki67) than tamoxifen. Anastrozole, Exemestane, and Letrozole have all demonstrated similar effect on proliferation indices. One study showed better clinical response rates with letrozole than tamoxifen.14 For these reasons, aromatase inhibitors are preferred in menopausal women, although tamoxifen can be used in patients with contraindications or intolerance to AIs.
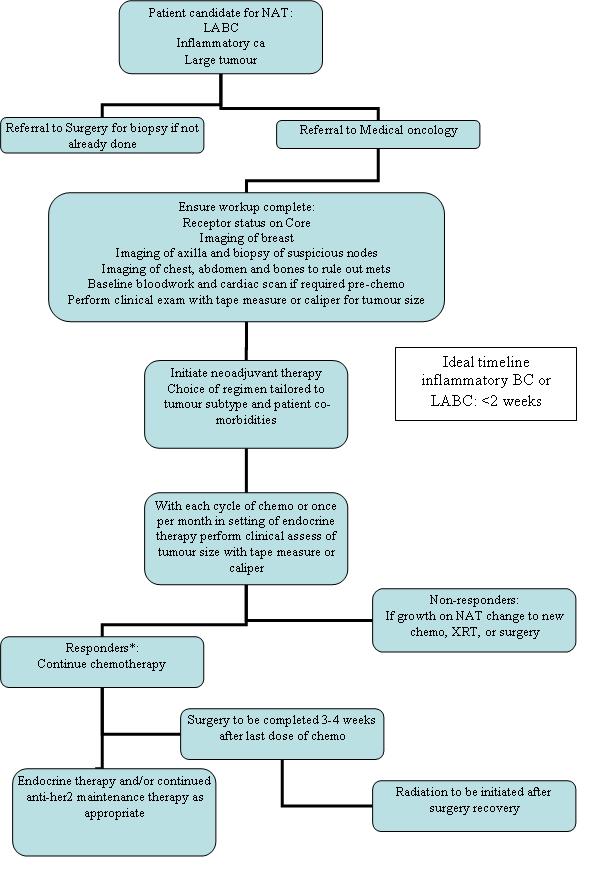
The above guideline can be summarized in the following pathway of care:
References
- Chia S, Swain SM, Byrd DR, et al. Locally advanced and inflammatory breast cancer. J Clin Oncol 2008: 25(5):786-90.
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96-102.
- Simmons CE, Hogeveen S, Leonard R, Rajmohan Y, Han D, Wong A, et al. A Canadian national expert consensus on neoadjuvant therapy for breast cancer: linking practice to evidence and beyond. Curr Oncol. 2015 Mar;22(Suppl 1):S43-53
- http://www.current-oncology.com/index.php/oncology/article/view/2328/1692
- Neo-adjuvant (Pre-Operative) Therapy for Breast Cancer – General Considerations, Clinical Practice Guidelines, Alberta Health Services, Alberta, Canada. Effective: December 2014. http://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-br015.pdf
- Brackstone M, Fletcher GG, Dayes IS, Madarnas Y, Sengupta SK, Verma S, et al. Locoregional therapy of locally advanced breast cancer: a clinical practice guideline. Curr Oncol, 2015;22(Suppl 1):S54-66.
- https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=334821
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary Breast Cancer: ESMO Clinical Practice Guidelines. Ann Oncol (2015) 26 (suppl 5): v8-v30. http://www.esmo.org/Guidelines/Breast-Cancer/Primary-Breast-Cancer
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 2.2016). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Kaufmann M, von Minckwitz G, Eleftherios P, Cameron D, Carey L, Cristofanilli M et al. Recommendations from the International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann Surg Oncol (2012) 19:1508–1516
- Teshome M, Hunt KK. Neoadjuvant Therapy in the Treatment of Breast Cancer. Surg Oncol Clin N Am. 2014 July ; 23(3): 505–523
- Kaufmann M, von Minckwitz G, Mamounas EP,et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012 May;19(5):1508-16.
- Cortazar P, Zhang L, Untch M, et al. Meta-Analysis results form the Collaborative Trials in Neoadjuvant Breast cancer (CTNeoBC). Cancer Research 72 (24) supplement 2012: S1-11
- Shenkier, T., et al., Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. CMAJ, 2004. 170(6): 983-994.
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant Treatment of Postmenopausal Breast Cancer With Anastrozole, Tamoxifen, or Both in Combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined With Tamoxifen (IMPACT) Multicenter Double-Blind Randomized Trial. J Clin Oncol August 1, 2005 vol. 23 no. 22: 5108-5116
- Eiermann W, Paepke S, Appfelstaedt, J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12(11):1527-32.

