Revised 29 November 2021
The tumour stage may be clinical (c) or pathological (p). A “yp” stage refers to tumours treated with pre-operative chemo-, radiation-, or chemo-radiation therapy which has the potential effect of “downstaging” a tumour that was previously classified as a higher clinical stage. In general, adjuvant therapy decisions are based on clinical stage with stage 0/I rectal tumours treated with surgery alone and stage II/III tumours treated with surgery, pre-operative radiation/chemoradiation and post-operative chemotherapy. Studies are underway to identify patients that may not require tri-modality therapy.
Stage 0
Cancer is limited to mucosa without invasion of the lamina propria
- Local, transanal excision or endoscopic polypectomy with clear margins.
- Transabdominal resection (Anterior Resection, Low Anterior Resection, Abdomino Perineal Resection) for larger lesions not amenable to local excision.
Stage I: T1-2, N0, M0
- Transabdominal resection (AR, LAR, APR) with no requirement for neoadjuvant or adjuvant radiation therapy remains standard of care.
- In patients whose medical comorbidities preclude formal transabdominal resection or who refuse major surgery despite counseling, local, transanal excision may be considered on a case by case basis. It is recommended that treatment with local excision only (for example Transanal Endoscopic Microsurgery) be offered in experienced centres only and should consider clinical as well as pathologic criteria (T1 lesion, well or moderate differentiation, size less than 3 cm, and absence of lymphovascular or perineural invasion, node-negative on preoperative imaging, and ability to achieve negative deep margin of at least 3mm). Postoperative radiation therapy is generally considered after local excision has been carried out.
Stage II: T3, N0, M0
- Referral to Radiation/Medical Oncology for neoadjuvant (preoperative) radiation therapy (with or without chemotherapy) is recommended.
- For non-fixed tumours in the upper two-thirds of the rectum: neoadjuvant short-course radiation therapy (2500cGy in five fractions) to be followed by LAR or APR within approximately ten days of the first day of radiation. (T3N0 tumours in the upper third of the rectum that have predicted clear radial margins may not benefit from radiation).
- For any fixed rectal tumours, rectal tumours approaching the mesorectal margin, and for tumours in the distal third of rectum: neoadjuvant long-course radiation therapy (4500cGy in 25 fractions with a possible boost of 540 cGy in 3 fractions) with concurrent chemotherapy to be followed by LAR or APR six to ten weeks following the completion of neoadjuvant therapy.
- Adjuvant capecitabine is given for 6 months following short-course radiation therapy (GIRCAP) or for 4 months following long-course chemoradiation therapy (GIRCRT).
- Adjuvant chemotherapy should start as close to four weeks post-op as possible.
- Patients treated with upfront resection and with pathologic Stage II rectal cancer should be referred to BC Cancer medical oncology and radiation oncology for postoperative adjuvant chemotherapy and radiation treatment (GIRCRT).
- See Stage III adjuvant chemotherapy recommendations for patients with ypN+ disease
- Consider treatment in a clinical trial, if available.
Stage III or T4NX: T4 or Tany, N+1,M0
- Referral to Radiation/Medical Oncology for neoadjuvant (preoperative) radiation therapy (with or without chemotherapy) is recommended.
- For mobile, non-fixed tumours in the upper two-thirds of the rectum: neoadjuvant short-course radiation therapy (2500cGy in five fractions of the first day of radiation) to be followed by LAR or APR within approximately ten days of the first day of radiation. Long-course chemoradiotherapy may also be used.
- For any fixed rectal tumours, rectal tumours or lymph nodes approaching the mesorectal margin, and for tumours in the distal third of rectum: neoadjuvant long-course radiation therapy (4500cGy in 25 fractions with a possible boost of 540 cGy in 3 fractions) with or without concurrent chemotherapy to be followed by LAR or APR six to ten weeks following the completion of neoadjuvant therapy.
- Adjuvant chemotherapy is given for 6 months following short-course radiation therapy or for 4 months following long-course chemoradiation therapy. Standard of care options include capecitabine or modified FOLFOX6. Current evidence does not specify optimal post-operative systemic therapy. There is some evidence that patients who achieve good downstaging (T0-2, N0) benefit from adjuvant 5-FU based therapy. Capecitabine is recommended for patients with ypT0-2N0 disease.
- mFOLFOX6 is considered for patients with ypTanyN+ disease as these patients are known to be at high risk of distant relapse.
- Patients presenting with advanced clinical stage (bulky T or N stage, T4 or N2) who achieve good downstaging may be considered for either capecitabine or FOLFOX.
- Please refer to current treatment protocols for indications, dosing and eligibility criteria.
- Adjuvant chemotherapy should start as close to four weeks post-op as possible.
- Patients treated with upfront resection and with pathologic Stage III rectal cancer should be referred to BC Cancer medical oncology and radiation oncology for postoperative adjuvant chemotherapy and radiation treatment (GIRCRT).
- Consider treatment in a clinical trial, if available.
Total Neoadjuvant Therapy (TNT) for Rectal Cancer
- TNT is an evolving approach for rectal cancers and can be considered as one of the standards of care. Several clinical trials have studied different approaches for rectal cancer and a consensus statement from the Western GI Consensus has been produced.
- All patients should be discussed at multidisciplinary rounds. A staging MRI should be performed and reported in a synoptic standard. It can be used for patients with good performance status with T4, N2 disease, EMVI, involved mesorectal fascia and/or enlarged lateral lymph nodes and patients with low rectal cancers at risk for APR. It can be used in select cases for patients with less bulky disease.
- Fluoropyrimidine and oxaliplatin based chemotherapy is the favoured systemic therapy approach in TNT and in select cases the addition of irinotecan can be considered. Both long course and short course radiation are reasonable options in the TNT approach and may be tailored based on tumour and patient factors. Four months of chemotherapy is recommended.
- The optimal sequencing can be short course or long course chemoradiation prior to consolidative chemotherapy as this leads to improved pathologic complete response (CR).
Summary:
- Long course chemoradiation with capecitabine or short course radiation followed by 4 months of chemotherapy approximately 2 weeks after completion of radiation.
- Patients should undergo standard oncologic surgery for rectal cancer with the standard surveillance strategy. In the cases of a CR, non-operative management with a surveillance strategy can be considered in the context of a clinical trial. See table 1.
- Long term data is still pending and a final approach is still to be determined.
Non Operative Management (Active Surveillance
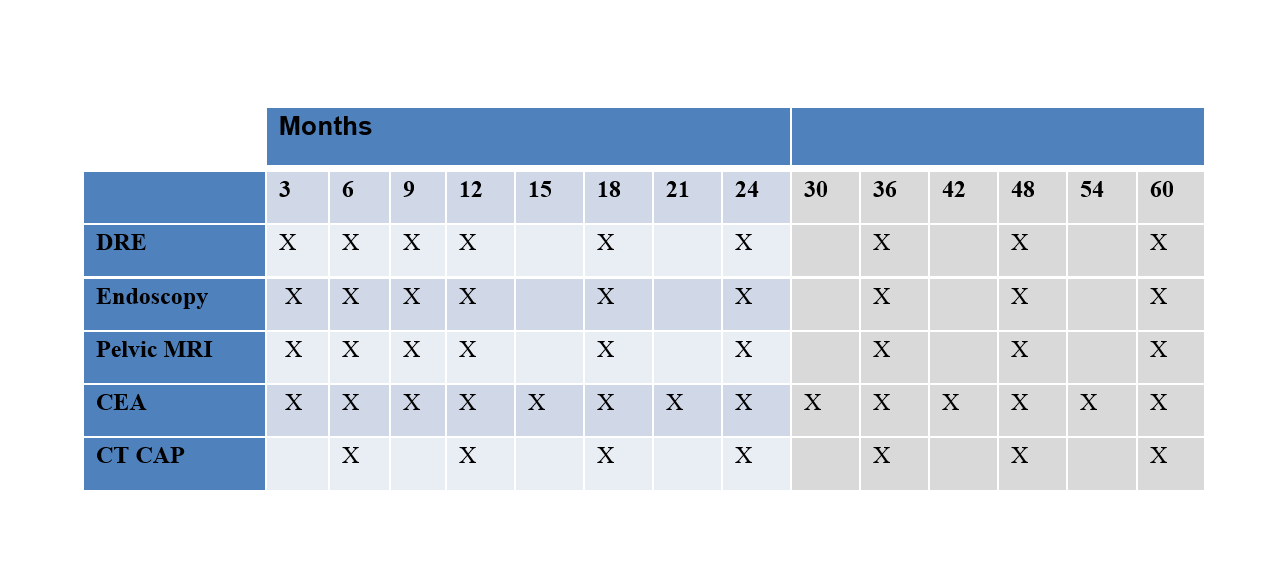
Table 1 Active Surveillance Schedule
Assessment for Local Re-growth
Regular assessments for local re-growth will be performed by the treating surgeon and will consist of DRE, endoscopy and MRI as per the schedule in Table 1. If any of these investigations do not meet criteria for cCR or if there is any uncertainty about these findings, this will be considered a local re-growth. Biopsies of the local re-growth will be required, however, surgery will be recommended for both positive and negative biopsy results (due to concerns about false negative results).
Assessment for Metastatic Disease
Regular assessments for metastatic disease will be performed by the treating medical oncologist and will consist of CEA and CT CAP as per the schedule in Table 1. If at any time point the CEA is elevated, this will prompt a CT CAP if this has not already been done.
Local Excision for Early Rectal Cancer
In select patients with early stage rectal cancer, a reasonable alternative to radical resection is combination neoadjuvant therapy followed by transanal endoscopic surgery (TES).
Patients considered for this approach should be discussed at multidisciplinary conference. Surgery for these patients should be provided at a high volume centre where >20 TES procedures are performed per year.
Patients should be counselled about the increased risk (10-15%) of local recurrence but similar 5 year disease free survival. These patients should be followed by an intensive surveillance program if medically fit for early salvage surgery.
POST TES (Active Surveillance)
Table 2 Active Surveillance Schedule
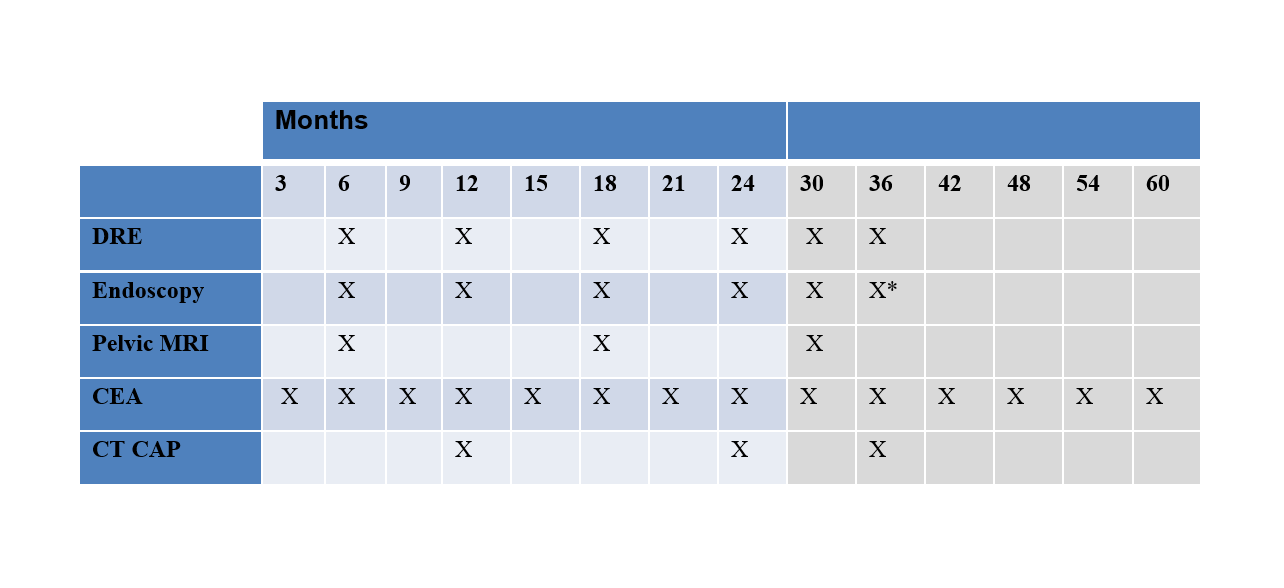
- Endoscopy after 36 months would be at the discretion of the endoscopist
Patients should be considered for a clinical trial.
Assessment for Local Re-growth
Regular assessments for local re-growth will be performed by the treating surgeon and will consist of DRE, endoscopy and MRI as per the schedule in Table 2. If any of these investigations do not meet criteria for cCR or if there is any uncertainty about these findings, this will be considered a local re-growth. Biopsies of the local re-growth will be required, however, surgery will be recommended for both positive and negative biopsy results (due to concerns about false negative results).
Assessment for Metastatic Disease
Regular assessments for metastatic disease will be performed by the treating medical oncologist and will consist of CEA and CT CAP as per the schedule in Table 2. If at any time point the CEA is elevated, this will prompt a CT CAP if this has not already been done.
Stage IV: any T, any N, M1
- In spite of the presence of metastases, excision of the primary tumour may provide the best form of local palliation. In appropriate circumstances, operation may be facilitated by preoperative chemotherapy and radiation.
- Locoregional/organ specific directed therapy may be considered for patients with isolated liver or lung metastasis. Referral to BC Cancer for multi-disciplinary assessment is recommended.
- Palliative radiation therapy or stereotactic body radiation therapy (SBRT) in selected patients; referral of SBRT patients to BC Cancer for multi-disciplinary assessment is recommended.
- Palliative chemotherapy has been shown to significantly improve survival in patients with unresectable metastatic colorectal cancer achieving an estimated median overall survival of 24 to 28 months as compared to 6 to 12 months with supportive care alone. Currently approved chemotherapeutic agents for metastatic colon cancer include: capecitabine, 5-fluorouracil (5-FU), irinotecan and oxaliplatin.
- The most commonly used regimens are:
- oxaliplatin with 5-FU (FOLFOX) or capecitabine (CAPOX)
- irinotecan with 5-FU (FOLFIRI) or capecitabine (CAPIRI)
- capecitabine monotherapy
- The choice and sequence of chemotherapy is determined by disease-related factors, patient factors and patient preferences as assessed by the medical oncologist.
- Currently approved targeted therapies include: bevacizumab, cetuximab, and panitumumab.
- Bevacizumab is recommended for use in combination with first-line FOLFIRI chemotherapy (FOLFOX may be considered in selected circumstances). It may also be considered for use in combination with second-line doublet chemotherapy for those patients who did not receive it in first-line.
- Cetuximab or panitumumab is indicated in patients with previously treated metastatic colorectal cancer with wild-type K-ras gene (i.e. tumour K-ras gene is not mutated).
- Please refer to current treatment protocols for indications, dosing and eligibility criteria.
- Consider treatment on a clinical trial, if available.
- Symptom management, best supportive care, and involvement of palliative care services as indicated by patient’s clinical status.
