In a setting of infinite resources and OR access, all EC patients could be managed in tertiary cancer centres and managed by gynecologic oncologists, but given current realities/access to ORs, intelligent triage is essential. These recommendations are meant to direct who can safely and appropriately be managed in the community by general gynecologists and where it is anticipated that management would be no different e.g. no justification for lymph node assessment or more complicated staging, low risk of second surgery, high likelihood of curative surgery.
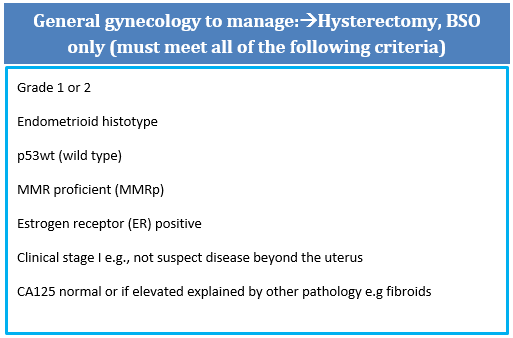
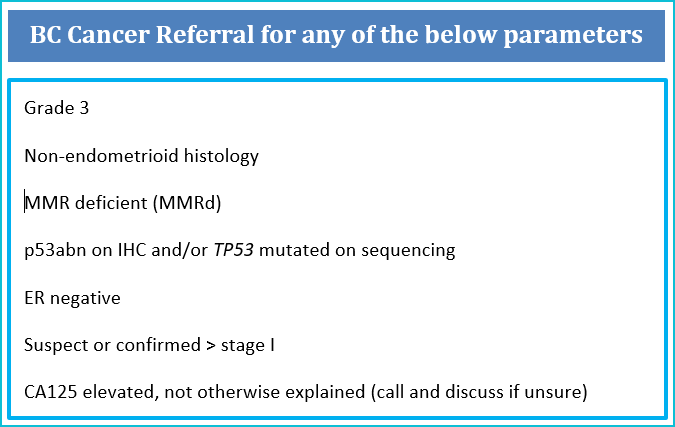
The rational for this referral approach in endometrial carcinoma (EC) includes:
- Grade 1/2 binary grading is more reproducible than 3-tiered EC grading and better reflects behaviour e.g. low grade (Gr1/2) vs high grade (G3) and is recommended by the WHO
- p53abn ECs are more aggressive and with higher risk (~ 45%) of lymph node metastases (LNM) (1)
- MMRd tumours have a higher likelihood of LVI and some MMRd tumours (e.g. with MLH1 loss) are more likely to have LNM and worse outcomes (2)
- p53wt, MMR proficient and estrogen receptor positive tumours that are clinically stage I/confined to uterus have excellent outcomes whether node negative or nodes not tested suggesting lymph node dissection for clinically negative or occult nodes is not therapeutic (therefore patients can be managed in community without lymph node assessment) (3-4)
- MMR proficient, p53wt and estrogen receptor positive tumours that are early stage have excellent prognoses, often not needing any adjuvant therapies and are appropriately followed in the community (3-4)
NB All components of this pathology criteria for referral (grade, histotype, MMR IHC, p53 IHC, and ER IHC) can and should be done in your local centre pathology laboratory and are considered standard of care in BC.
Clinical Stage II (bulky cervical involvement) that is not amendable to surgical resection
Clinical State IIIC (with bulky and unresectable nodes)
Clinical Stage IV (widespread metastatic disease on preoperative imaging, or bladder/bowel involvement)
We recommend all patients with advanced disease be presented at a multidisciplinary tumour conference to review optimal management strategies. Many patients will receive neoadjuvant chemotherapy, followed by repeat imaging and assessment by a gynecologic oncologist after the 3rd cycle of chemotherapy to determine response to treatment and suitability for surgery. If there has been a good radiological response to treatment, surgery will usually be considered after the 3rd cycle of chemotherapy. This will be followed by post-operative chemotherapy usually to a total of 6 cycles, with or without radiotherapy, to be determined by the multidisciplinary team (see sections on adjuvant therapies).
- A Jamieson, E Thompson, J Huvila et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases.
Gynecol Oncol. 2022 Mar 2.
https://doi.org/10.1016/j.ygyno.2022.01.025. Epub ahead of print. PMID 35504673
- Post CCB, Stelloo E, Smit VTHBM, et al. Prevalence and Prognosis of Lynch Syndrome and Sporadic Mismatch Repair Deficiency in Endometrial Cancer.
J Natl Cancer Inst; https://do.
- Amy Jamieson, Naveena Singh, Jutta Huvila et al. The continuing evolution of endometrial carcinoma molecular classification: risk stratification within the No Specific Molecular Profile (NSMP) subtype. Gynecol Oncol, Jan 2023
https://doi.org/10.1016/j.ygyno.2022.12.01
- A Jamieson, J. Huvila, D. Chiu et al. Grade and estrogen receptor expression identify a subset of No Specific Molecular Profile (NSMP) endometrial carcinomas at very low risk of disease-specific death.
Mod Pathol, Jan 2023
https://doi.org/10.1016/j.modpat.2022.100085
- Vermij, L., Jobsen, J.J., León-Castillo, A. et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry.
Br J Cancer (2023). https://doi.org/10.1038/s41416-023-02141-0
The prognostic value of molecular classification has been demonstrated repeatedly; across unselected population-based series, clinical trials, and even narrowly defined age- or histotype-stratified subsets. Specifically, patients with
POLEmut EC have highly favorable outcomes with almost no recurrence events, in contrast to p53abn EC where >50% of patients recur and die from their disease. MMRd and NSMP have intermediate outcomes and need further prognostic refinement. Recent data also supports the predictive implications of molecular subtype assignment. Molecular sub-group analysis of clinical trial data (PORTEC-3 trial (1)) and a large retrospective series (cohort of 2500 ECs (2)) have both demonstrated improved survival outcomes in patients with p53abn ECs with adjuvant platinum-based chemotherapy when used with radiation compared to radiation alone in patients with European Society of Medical Oncology (ESMO) defined high risk EC. In contrast, there was no apparent benefit of chemotherapy observed in these series for patients with high risk MMRd ECs, compared to radiation alone. Individual patient data meta-analysis of all published and available international reports on
POLEmut ECs showed that patients with confirmed pathogenic
POLE mutations have almost no recurrence or disease specific death events, even when their tumours appear to have unfavourable clinicopathological or molecular characteristics, and adjuvant treatment was not associated with outcomes (3). There are a growing number of molecular subtype-specific clinical trials and ALL EC patients should be assessed for trial eligibility. For patients not eligible for clinical trials, it is recommended to follow this molecular directed adjuvant therapy algorithm.
This algorithm should be applied to all surgically resected ECs of any histotype, including non-endometrioid carcinomas.
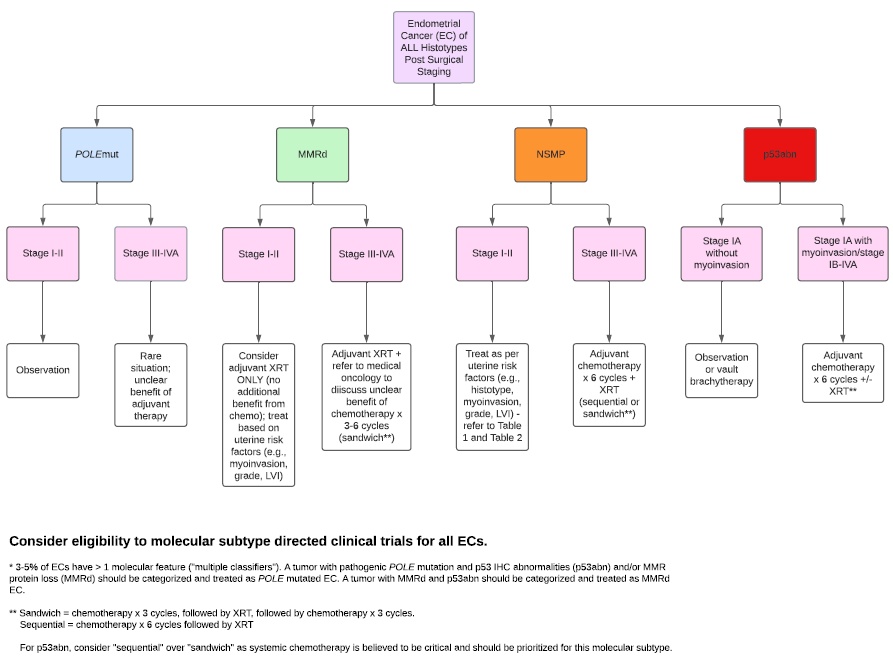
Adjuvant chemotherapy
Carboplatin and paclitaxel 3 weekly schedule (GOENDCAT) is the recommended protocol) for 4- 6 cycles. If a patient has intolerance to GOENDCAT, consideration should be given to another taxane-platinum regimen. Carboplatin/liposomal doxorubicin (GOOVPLDC) can be considered if there is a contraindication to all taxanes.
Table 1. Adjuvant therapy guidelines for NSMP endometrioid carcinomas:
| |
IA |
IB |
II** |
IIIA /B |
IIIC |
IV*** |
| Grade 1 | O* | VB | C, EB+/-VB | C+EB | C+EB | C |
| Grade 2 | O* | VB | C, EB +/- VB | C+EB | C+EB | C |
| Grade 3 | VB | C+EB | C, EB+/-VB | C+EB | C+EB | C |
- O=observation, VB=vault brachytherapy, EB=external beam radiation, C=chemotherapy
NB There is no level 1 evidence showing benefit from adjuvant chemotherapy used in addition to radiation in stage I-II NSMP ECs. We recommended referral for a discussion regarding adjuvant chemotherapy when indicated in table 1.
* For Stage IA or IB recommend pelvic radiotherapy if there is
extensive/substantial LVSI (≥3 foci)
**For Stage II, chemotherapy should be considered if there is at least one additional high-risk uterine factor (deep myometrial invasion, or grade 3 tumour, or extensive LVSI).
*** Hormonal therapy, can be considered as first-line therapy in carefully selected stage IVB grade 1-2 tumours if ER positive (see section 9. Advanced Endometrial Cancer).
Table 2. Adjuvant therapy guidelines for NSMP non-endometrioid carcinomas:
| STAGE | IA M- | IA M+ | IB, II, III | IV |
| Fully staged* | Observation or VB | C+VB | C+EB | C |
| Incompletely staged | Recommend restaging | C+EB | C+EB | C |
- M–: no myometrial invasion; M+: myometrial invasion
- *Fully staged includes lymph node assessment, omentectomy, washing
NB There is no level 1 evidence showing benefit from adjuvant chemotherapy used in addition to radiation in stage I-II NSMP ECs. We recommended referral for a discussion regarding adjuvant chemotherapy when indicated in table 2.
- León-Castillo A, de Boer SM, Powell ME, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy.
J Clin Oncol 2020; JCO.20.00549.
- Amy Jamieson, Jutta Huvila, Samuel Leung et al. Molecular subtype stratified outcomes according to adjuvant therapy in endometrial cancer. Gynecol Oncol Jan 2023 PMID: 36753816 DOI: 10.1016/j.ygyno.2023.01.025
- McAlpine JN, Chiu DS, Nout RA, et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis.
Cancer 2021; 1–14.
NB MMR and p53 immunohistochemistry are available in any pathology laboratory and is now considered standard of care for all ECs in BC.
POLE testing has been approved in BC since May 2022. Testing in the current funding scheme is being paid for, for patients in whom information on
POLE status would impact adjuvant treatment decisions (see
POLE testing section below).
Mismatch repair testing
Mismatch repair is a DNA repair pathway. Loss of function of mismatch repair enzymes can arise secondary to inherited susceptibilities (e.g Lynch Syndrome), can arise sporadically through somatic mutation or can be epigenetic where a key protein associated with mismatch repair (e.g. MLH1) is not present because of selective hypermethylation of the genome in a way that turns off translation of the gene. Mismatch repair is usually measured in BC by IHC testing for two proteins: PMS2 and MSH6. Testing is performed on tumour specimens—biopsy or hysterectomy (not needed on both) and loss of one or more of these proteins (with a positive internal control) is termed mismatch repair deficient. You may see comments on pathology reports that status of other mismatch repair proteins cannot be determined. This is because PMS2- MLH1 and MSH2- MSH6 form heterodimers and loss of one can cause weak or absent expression of the other. All you need to know as a clinician is 1) loss of expression of one or more MMR proteins means the case has been categorized as MMRd (sometimes also referred to as MMR loss or MMR abnormal), 2) HCP referral should be initiated if the carcinoma is MMRd UNLESS hypermethylation of MLH1 is identified. Note: methylation testing can take 4-8 weeks so referral to hereditary cancer program testing in the interim is recommended and this should be cancelled if hypermethylation is identified.
Mismatch repair deficiency may be identified by 4 protein IHC (loss of one or more defined mismatch repair deficient) or by DNA-based microsatellite instability (MSI) assay, but this is less common in BC.
p53 testing
p53 is a protein also commonly assessed by IHC in multiple gynecologic cancers, and is a key part of molecular classification of endometrial cancers. Criteria for categorization of p53 abnormalities by IHC differ slightly between tumour sites but in general, abnormal p53 expression (p53abn) encompasses three scenarios; i) complete loss of protein or ii) overexpression or iii) subclonal/zonal expression (distinct and regional abnormal staining). Normal p53 expression levels as measured by IHC (or
TP53 mutation status on a next generation sequencing panel), is termed 'wild type' or p53wt.
POLE
testing
POLE is a gene also involved in DNA repair and when specific pathogenic mutations are identified in the
POLE gene this identifies a subset of EC patients at very low risk of recurrence and death from their disease. These patients may be candidates for de-escalated adjuvant therapy in clinical trials or as recommended by their physicians according to BC and international guidelines.
POLE testing has been approved in BC since May 2022. Testing in the current funding scheme is being paid for but ordered after hysterectomy for those patients in whom information on
POLE status would impact adjuvant treatment decisions. It may be ordered reflexively by the pathologist or, more commonly, is ordered by the treating oncologist when needed (see table below). Click
here for POLE requisition.

Correct interpretation of molecular results
Clarification of pathogenicity of
POLE mutation is imperative, with pathogenic list of mutations considered 'actionable' for
POLEmut assignment currently limited to 11 well characterized missense mutations (P286R, V411L, S297F, S459F, A456P, F367S, L424I, M295R, P436R, M444K, D368Y) (1).
Multiple-Classifier Positive Cases
How to manage the approximately 3-5% of ECs with more than one molecular feature (called multiple-classifiers) is also crucial.
TP53 mutations can occur as secondary events in ultramutated
POLEmut ECs and/or hypermutated MMRd ECs, with molecular features and clinical behaviour of
POLEmut and MMRd ECs, respectively (2). These findings suggest that these secondary
TP53 mutations are a later event acquired during tumour progression that do not affect the clinical outcome. These tumours should be managed according to the WHO and ProMisE order of segregation, with any EC harbouring a pathogenic
POLE mutation considered
POLEmut (even if p53abn and/or MMR abnormal IHC) and next any EC with loss of MMR proteins or MSI considered MMRd even if p53abn/TP53mut detected (3).
- León-Castillo A, Britton H, McConechy MK, et al. Interpretation of somatic POLE mutations in endometrial carcinoma.
J Pathol. 2020;250(3):323-335. doi:10.1002/path.5372
- León-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of 'multiple-classifier' endometrial carcinomas.
J Pathol. 2020;250(3):312-322. doi:10.1002/path.5373
- WHO Classification of Tumours. Female genital tumours: International Agency for Research on Cancer Lyon (France): International Agency for Research on Cancer; 2020.
Treatment of recurrent endometrial cancer will depend on sites of recurrence and primary treatment.
In the setting of isolated disease in the absence of previous adjuvant therapy, cure may be possible with radiotherapy or surgery for isolated vaginal vault or pelvic disease. These patients should be referred to BC Cancer. Radiotherapy is generally reserved for those with localized recurrences, while chemotherapy is offered to those with distant disease. Pelvic exenteration may be considered for those who recur within a radiated field, when the recurrence is localized to the central pelvis and there is no evidence of distant disease based on PET/CT.
First-Line Therapy
In the upfront treatment setting, combination chemotherapy with carboplatin and a taxane is the current standard of care. Treatment is usually for 6 cycles (GOENDCAT or GOENDCAD).
Second-Line Therapy - MMRd
For patients with MMRd endometrial cancer that has progressed after first-line chemotherapy, treatment with immune checkpoint inhibitors has been demonstrated to have a high response rate and long durations of treatment benefit. Access to immune checkpoint inhibitors is possible through patient access programs, and this can be determined by contacting the regional Drug Access Navigator (1-2).
Second-Line Therapy - MMRp
For patients with MMRp endometrial cancer that has progressed after first-line chemotherapy, a phase 3 clinical trial has demonstrated that the use of lenvatinib and pembrolizumab improves progression-free survival and overall survival (3). Access to this combination is currently possible through a patient access program. Contact your regional Drug Access Navigator for additional information.
Other treatment options
Grade 1 (and possibly grade 2) histology or significant comorbidities precluding chemotherapy- consider hormonal therapy if tumour is ER or PR positive (GOENDAI or GOENDH). Early initiation of palliative care/supportive care is recommended with or without chemotherapy. Radiation therapy may be considered for symptomatic disease (e.g. pain, bleeding).
1. Oaknin A, et al. Safety and antitumour activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022 Jan;10(1):e003777. doi: 10.1136/jitc-2021-003777. PMID: 35064011; PMCID: PMC8785197.
2. O'Malley DM, et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J Clin Oncol. 2022 Mar 1;40(7):752-761. doi: 10.1200/JCO.21.01874. Epub 2022 Jan 6. PMID: 34990208; PMCID: PMC8887941.
3. Makker V et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022 Feb 3;386(5):437-448. doi: 10.1056/NEJMoa2108330. Epub 2022 Jan 19. PMID: 35045221.
Endometrial tumours that develop on estrogen replacement therapy tend to be well differentiated and minimally invasive, and therefore these women generally have a good prognosis. However, the known association between estrogen and EC causes concern when women with a history of EC request hormone replacement. Many of these patients are asymptomatic. There is a small proportion of patients (usually premenopausal) who suffer from extreme vasomotor symptoms. Unfortunately, there are limited non-hormonal alternatives to estrogen, such as clonidine or venlafaxine. For premenopausal women, thorough discussion of the risks and benefits of estrogen replacement is essential. The absence of hormone replacement therapy in young women who have surgery including bilateral salpingo-oophorectomy before age 50 is known to be associated with an increased risk of death secondary to coronary heart disease, osteoporosis, thromboembolic events, and cancers such as lung and colorectal cancer (1). There is no evidence that estrogen replacement therapy use alters the prognosis in women who have had treatment for endometrial cancer (2).
- Parker WH et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses' Health Study. Obstet Gynecol 2013;121(4):709-16.
- Barakat RR et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in Stage I or II endometrial cancer: A Gynecologic Oncology Group study J Clin Oncol 2006;1;24(4):587-92.
