Multi-modality management of locally advanced NSCLC continues to be associated with controversy and practice variations for the role of surgery, radiation oncology and medical oncology. In large measure, this stems from the fact that this patient population involves a broad range of disease burden, from patients with extensive mediastinal involvement, others with discrete mediastinal involvement, and those with incidental lymph node involvement discovered when lymph nodes are examined after a resection with negative mediastinal staging pre-operatively. Additionally, the controlled clinical trials examining issues of treatment are complex, often underpowered and associated with some disagreement of interpretation. It is difficult to compare results across trials as characterization of the patients included is variable (i.e. clinical vs. pathologic staging, thoroughness of staging with PET imaging) and it is unclear whether the patient populations are comparable. Nevertheless, progress has been made and areas of consensus have emerged. Unavoidably, locally advanced NSCLC management guidelines from various groups do have some differences reflecting the opinions and treatment philosophy of the physicians involved in their generation.
Relevant TNM Stage Groups
The locally advanced non-small cell lung cancer (LANSCLC) stage groupings include stage III patients (any T N2-N3M0, T4N0M0, and T3N1M0) and those stage II patients (T2B-T3NOM0 and T1-2N1M0 who cannot undergo a definitive resection due to medical inoperability. Locally advanced NSCLC makes up about 30-35% of the NSCLC patient population.1
Patient Selection for Combined Modality Therapy
Stage III NSCLC patients appropriate for combined modality therapy should have good performance status (ECOG 0 or 1) and minimum weight loss (less than 5% in the preceding three months). The importance of these simple criteria is that when patients are chosen that violate these rules, analysis of outcome shows that they do not benefit as much from intensive therapy. Additionally, patients with poor performance status and weight loss have a high risk of serious complications including treatment related death from toxicity. In carefully selected patients with ECOG PS 2 or weight loss up to 10%, concurrent chemoradiation may be a consideration if the attending consultants are mutually agreed that this is appropriate. Patient-related and tumour-related factors can influence the balance of risks vs benefits; patient preferences should also play a significant role.
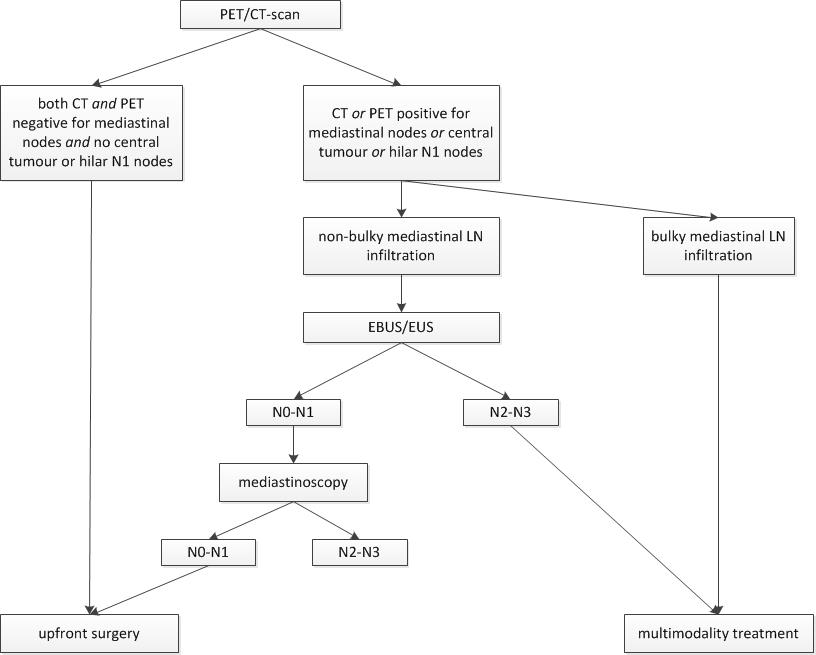
Because of the intensive nature of the treatment for potentially curable locally advanced lung cancer, staging must be precise to avoid aggressive combined modality treatment in a patient that is incurable with metastases. In addition to CT scanning, positron emission tomography (PET) should be done routinely to exclude distant metastases and help define the disease in the thorax requiring radiotherapy. A favourable distribution of thoracic disease for definitive local therapy (radiotherapy/surgery) is a major factor.1 Additionally, many patients will require pathologic assessment of the lymph node stations by endobronchial ultrasound biopsy (EBUS) or mediastinoscopy to minimize false positive or negative CT and PET scan results.
Patients with locally advanced NSCLC have high risk disease but when treatment can be given with curative intent, it is necessary to organize investigation and treatment in a timely fashion. Rapid disease progression may occur with delays as has been documented by Mohammed et al.2 Using serial PET and CT scans, the authors showed 3% of untreated patients developed distant metastases at 4 weeks and 13% at 8 weeks. Restaging should be considered if there is a delay of 8 weeks from the most recent CT scan. Thus, delay not only impacts on resource utilization but directly on patient outcome.
Although age is not a clear selection criteria, generally speaking, patients over 75 years of age must be very fit and motivated to receive multi-modality therapy. Co-morbid conditions impact treatment selection for all disciplines. Definitive local therapy with surgery and thoracic irradiation requires adequate pulmonary function. Standard chemotherapy regimens require adequate hematologic, hepatic and renal function.
It is crucial that all treating oncologists (thoracic surgeons, radiation oncologists and medical oncologists) mutually agree on the proposed treatment plan and the suitability of the individual patient for such therapy before any component of treatment has commenced. Such cases should be presented at a multi-disciplinary lung conference. Of all patients with locally advanced NSCLC, a minority rather than a majority will be suitable for aggressive treatment with curative intent.
Active patient participation in decision making respects the fundamental ethical and legal doctrine of autonomy, while exploring the concerns of these individuals, including functional limitations, symptoms and side effects/risks of therapy. Patients should have some idea of the quantity of median and long-term survival gain expected with intensive therapy to make an informed choice on the pros and cons of a conservative versus a more aggressive treatment program. Unfortunately, the majority of locally advanced NSCLC patients will experience an incurable relapse and it is unfair for them to go into a complex and toxic treatment program under the false impression that the probability of cure is high. On the other hand, the gain in median survival and the increase in the proportion of long-term survivors with multi-modality therapy are sufficient that this arduous treatment should be recommended for appropriately selected patients.
Thoracic Radiotherapy Alone for Locally Advanced NSCLC
In patients with symptomatic infiltrative stage III (N2, N3) NSCLC and either performance status 3 or 4, comorbidities, or disease too extensive to treat with curative intent, palliative radiotherapy is recommended. The fractionation pattern should be chosen based on the physician’s judgment and patient needs.
In patients with stage III NSCLC with performance status of 0-1 and weight loss < 5%, radiotherapy alone is not recommended. Should the patient refuse multi-modality therapy, definitive radiotherapy alone with curative intent may be an alternative plan associated with a lower probability of long-term survival. Similarly, patients with co-morbid conditions, performance status of 2 and/or weight loss of 5-10% that are felt unsuitable for multi-modality therapy may be considered for definitive radiotherapy alone according to the radiation oncologist’s judgment.
Conventional thoracic radiotherapy alone in unresectable stage III NSCLC using conventional (2 Gy or equivalent to a total dose of 60 Gy) has been associated with a median survival of about 1 year and a 5-year survival of 5-7%.3
Combined Chemotherapy and Thoracic Irradiation for Locally Advanced NSCLC
With platinum containing chemotherapy regimens, investigators have improved survival by combining chemotherapy with thoracic irradiation in patients with good performance status and minimal weight loss. Three meta-analyses reviewing more than 50 trials confirm the survival benefit of combined platin-based chemotherapy with radiotherapy over radiotherapy alone in locally advanced, unresectable NSCLC.4,5,6
Optimal Sequencing of Chemotherapy and Thoracic Radiotherapy
Chemotherapy has been combined with radiotherapy in different ways (chemotherapy followed sequentially by radiotherapy, concurrent chemoradiation, induction chemotherapy followed by concurrent chemoradiation, or concurrent chemoradiation followed by consolidation chemotherapy). For curative intent treatment of locally advanced NSCLC, concurrent chemoradiation is recommended because it improves local control and overall survival compared with sequential chemotherapy followed by radiation or radiation alone. In multiple randomized trials and a meta-analysis (Auperin A. J Clin Oncol 28; 2181-2190) induction chemotherapy followed by radiotherapy (sequential chemoradiation) was compared to concurrent chemoradiation. Concurrent chemoradiation was associated with superior 5-year survival and better local control at the cost of a higher rate of reversible esophagitis.
The Chemotherapy Regimen
Few clinical trials in stage III disease have been specifically designed to investigate the optimal chemotherapy regimen for chemoradiation. The ideal concurrent chemotherapy regimen should be active against local intrathoracic disease and distant subclinical metastases, provide a radiosensitizing effect on tumour and combine with concurrent thoracic irradiation with manageable normal tissue toxicity. The two most common chemotherapy regimens are cisplatin/etoposide and carboplatin/paclitaxel. These regimens appear to perform in a similar fashion in a large Veterans Administration retrospective comparison (JCO 33: 567, 2015 and both regimens are available on BC Cancer chemoradiation protocols (LULAPE2RT, LULACART). A meta-analysis by Palma et al. reported that the low dose carboplatin/paclitaxel regimen is associated with a somewhat higher risk of pneumonitis (Palma D. Int J Rad Oncol Biol Physics 85: 444-450, 2013). Recently, a phase III trial comparing cisplatin/etoposide and cisplatin/pemetrexed (plus consolidation single agent pemetrexed) was stopped for futility with the demonstration that the pemetrexed containing regimen was not superior.7 The survival landmarks for concurrent chemoradiation for appropriately selected and staged patients is a median survival of 22-25 months and a 5 year survival of about 20% based on published randomized trials.7,8
No Role for Routine Induction and Consolidation Chemotherapy
The use of induction chemotherapy prior to concurrent chemoradiotherapy increases toxicity and was associated with no survival advantage.9 Similarly, there is no role for the routine use of consolidative chemotherapy after chemoradiotherapy as shown in a meta-analysis.10 This may be an option for patients who did not receive full systemic chemotherapy doses during radiotherapy. No form of targeted therapy has been shown to improve outcome for stage III NSCLC. Results of maintenance immunotherapy trials with checkpoint inhibitors given after chemoradiation are pending.
Role for Sequential Chemoradiation in Selected Cases
For patients who are not suitable for concurrent chemoradiation, sequential chemotherapy followed by radiotherapy is less efficacious but spatial separation of the modalities is less toxic. Such therapy may also be a consideration when radiotherapy treatment volumes are unduly large where downstaging may allow definitive radiotherapy. This approach may improve survival compared to radiotherapy alone.4
Radiotherapy Dose Fractionation and Technique
The standard dose fractionation of radiation with concurrent chemotherapy is 60 Gy given in fractions of 2 Gy once per day over 6 weeks.3,11,12 Dose escalation beyond 60 Gy with conventional fractionation has not been demonstrated to be of benefit.13 In RTOG 0617,13 74Gy was compared with 60Gy and was not found to be superior. Although the RTOG dose escalation study did not have toxicity as the primary endpoint, analysis of side effects suggests that planning radiotherapy with IMRT compared with 3DCRT allowed treatment of larger tumours with a lower risk of severe pneumonitis.
Combined Modality Therapy including Surgery for Stage III NSCLC
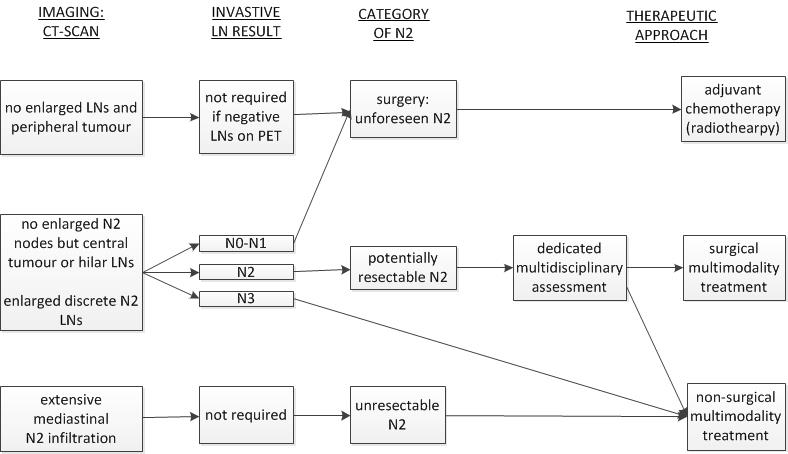
The surgical management of N2 stage IIIA disease is one of the most controversial topics in the management of NSCLC, especially if the disease appears “resectable”. Not all cases of N2 stage IIIA disease are equal. For the purposes of making surgical decisions, N2 stage IIIA NSCLC is further subdivided in 3 categories:
- Infiltrative N2 disease: These are patients with significant mediastinal involvement, such that individual lymph nodes can no longer be distinguished by CT. Included are also patient with mediastinal disease that partially or fully surrounds major mediastinal structures.
- Discrete N2 disease: These are patients with biopsy-proven N2 disease, but where individual lymph nodes are readily distinguishable on CT imaging. The lymph nodes may be normal sized (microscopic N2 disease) or enlarged on imaging, but they do not coalesce or invade major mediastinal structures
- Occult N2 disease: These are patients who are found to harbour N2 disease at resection, or on surgical pathology despite undergoing appropriate preoperative staging with CT, positron emission tomography (PET) and invasive mediastinal staging when indicated.
Surgical recommendations depend on category of N2 disease and the performance status of the patient. For the first group with infiltrative N2 disease, surgery is not recommended. Surgery plays a role in the second and third group of patients.
Occult N2 stage IIIA NSCLC after thorough preoperative staging
Due to improvements in preoperative staging modalities, this is a less common situation than in years past. There are no definitive guidelines on how to approach this problem. If surprise N2 disease is encountered at the time of thoracotomy, then most surgeons believe that proceeding with resection is reasonable since the risk of surgery has already been incurred by the patients. The patient would be considered for post-operative adjuvant chemotherapy with or without radiotherapy. If it is encountered during thoracoscopy, then one can terminate the procedure in favour of neo-adjuvant therapy and surgery or definitive chemoradiation. However, it is also valid to continue with the planned thoracoscopic resection and evaluate for adjuvant chemotherapy with or without radiotherapy. This decision is based on the extent of required resection, the ability to obtain free margins and the functional status of the patient.
Such patients should be seen in medical oncology consultation and considered for cisplatin-based adjuvant therapy. In the cisplatin-vinorelbine meta-analysis database, the long-term improvement in survival with such therapy for resected N2 positive patients was 15%.14 For patients with N2 lymph node involvement, post-operative radiotherapy may be recommended for patients with complete resection of N2 disease to improve local control but such therapy should be given sequentially after adjuvant chemotherapy.
Post-operative radiotherapy is recommended for patients with incomplete resection (microscopic or gross positive margin, or gross residual disease). Radiotherapy is to be given sequentially with chemotherapy for microscopic residual disease but for gross residual disease, concurrent chemoradiation may be considered for patients with good performance status after surgery.
Discrete N2 stage IIIA NSCLC
When involved N2 lymph nodes are identified pre-operatively by invasive mediastinal staging, surgery alone is not a recommended treatment option. Pre-operative chemotherapy followed by surgery has been investigated in randomized trials. A meta-analysis showed the absolute survival benefit at 5-years was 6% and downstaging to pT0 in the surgical specimen by chemotherapy was only 6-7%.16 Most patients were given thoracic irradiation after surgery because of unfavourable pathology reports. Protracted sequential deployment of chemotherapy followed by surgery followed by radiotherapy is not recommended.
A number of trials have examined pre-operative chemotherapy and radiotherapy after surgery.17,18,19 The results have not always showed benefit for tri-modality therapy versus chemoradiation alone, particularly when a series of neo-adjuvant chemotherapy treatments were given before pre-operative thoracic irradiation.18,19
There remains a persistent interest in the scientific community not to abandon surgery in stage III NSCLC because of the sub-optimal local control of the primary lung tumour achievable with thoracic radiotherapy or chemo-radiotherapy. Additionally, surgery provides the most definitive staging.
The Intergroup 0139 phase III randomized trial compared trimodality therapy (pre-operative concurrent chemoradiation (cisplatin/etoposide, 45 Gy) followed by surgery versus bimodality therapy with chemoradiation (61 Gy) has been influential on BC Cancer Lung Tumour Group Management Guidelines.17 There was no overall survival advantage to the trimodality arm (median survival 23.6 months versus 22.2 months) but progression-free survival was significantly improved (12.8 months versus 10.5 months). Overall, 88% of patients in the surgical arm were eligible for thoracotomy and 71% had complete surgical excision and only 4.5% had no resection. Notably, pathologic downstaging after chemoradiation showed pT0 in 20% and another 20% had only microscopic residual disease in the previously irradiated volume. Pathologic downstaging with pre-operative chemoradiation appears clearly superior to chemotherapy alone.16 Unfortunately, in this multicenter trial, experience with trimodality therapy was limited in some centres and serious toxicity problems occurred. Among those requiring pneumonectomy, post-operative mortality was 26%; it was 50% for complex right pneumonectomy. In an exploratory unplanned, matched subgroup analysis, patients treated with a lobectomy after induction concurrent chemoradiation had a significantly better survival than those judged suitable for lobectomy but treated non-surgically on the control arm.
While acknowledging the controversies in using trimodality therapy for stage III NSCLC, the BC Cancer Lung Cancer Lung Tumour Group endorses using it for a carefully selected stage III patient population. This is based on the fact that a number of groups20,21 including the BC Cancer Lung Tumour Group21 have shown that carefully selected patients may undergo trimodality therapy including pneumonectomy with low (2%) operative deaths. Our experience with trimodality therapy (cisplatin/etoposide concurrently with thoracic irradiation (45-60 Gy) followed by surgery has yielded median survival of over three years and 5-year survival of 35%. Although the favourable outcome is associated with careful selection as well as the treatment, the results are good enough to continue this policy, particularly when local thoracic surgery expertise has such consistent low morbidity and mortality rates over many years.
In summary, the key to optimal management of discrete N2 disease is concurrent chemotherapy and radiotherapy. Surgical resection has a role in carefully selected patients with limited burden of disease, good performance status, good pulmonary function and patient preference.
With regard to surgical therapy, even within the category of discrete N2 disease, there are subdivisions that have been studied in the literature that carry favourable or unfavourable characteristics. Groups that carry favourable prognosis include patients with complete pathologic response, ypN0 disease even with residual viable tumour in the primary site, patients requiring resection less than pneumonectomy, non-enlarged lymph nodes and single-station N2 disease. Although these groups of patients carry a better prognosis, it is difficult to make decisions on surgical recommendations based on their presence or absence. Many of these factors are not readily identifiable preoperatively, and hence cannot be used to guide decisions about surgery. In general, we do not use these criteria solely to make a decision about candidacy for surgery for discrete N2 disease, rather each piece of data is considered in the context of the individual patient. After discussion at a multidisciplinary lung tumour board, it is appropriate to consider all patients with good performance status and discrete N2 stage IIIA NSCLC for a strategy of pre-operative therapy followed by surgery. This includes patients with multi-station N2 disease and patients that may require pneumonectomy (assuming their pulmonary function allows for such a resection).
Trimodality therapy requires a dedicated multidisciplinary assessment with an experienced thoracic surgeon, radiation oncologist and medical oncologist. In cases of bulky locally advanced disease where the surgical treatment is uncertain, the radiation oncologist may prescribe 60 Gy rather than 45 Gy as the pre-operative radiotherapy prescription in case the surgical treatment is not recommended. A crucial step in this process is the surgeon’s assessment of resectability after concurrent chemoradiation. Imaging has typically been with CT. Repeat PET scanning has been proposed to identify patients where surgery would be futile.22 This has been associated with some controversy because of the high incidence of false positive results when PET is performed after chemoradiation.23 A comparison across studies suggests that the survival was better in those studies in which patients underwent resection despite the lack of PET response vs those studies in which the patients generally did not undergo resection. There is insufficient evidence to mandate repeat PET after induction chemoradiation to assess operability but it may be useful at the surgeon’s discretion in cases where there is an unsatisfactory CT response or suspicion of progression.
At this time, there is no evidence that additional chemotherapy or any type of targeted therapy is indicated after surgical resection in trimodality therapy, even if the pathology report is unfavourable.
Follow-Up
NSCLC patients treated with radical intent should be followed for treatment-related complications, detection of treatable relapse or occurrence of second primary lung cancer. There are no comparative studies that adequately define the most effective follow-up for patients with non-metastatic lung cancer. Surveillance is more guided by knowledge of relapse patterns, not by evidence that earlier detection of relapse and treatment leads to a better outcome.
At least 75% of relapses occur in the initial 2-3 years after treatment. Hence a follow-up visit every 3-6 months for the first 2-3 years followed by annual visits thereafter. History, physical examination, chest radiograph and annual CT scan are appropriate as the CT is the best way to detect a second primary tumour.
Smoking Cessation
Smoking is the main cause of lung cancer and smoking cessation is of major value of NSCLC patients, especially those treated with curative intent. It is associated with significantly decreased risks of mortality, development of a second primary lung cancer or recurrence.
References
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016 Jan;11(1):39-51.
- Mohammed N, Kestin LL, Grills IS, Battu M, Fitch DL, Wong CY, et al. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011 Feb 1;79(2):466-472.
- Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with recurrent unresected stage III NSCLC: RTOG 9410. ASCO; 2003.
- Pignon JP, Stewart LA. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb non-small cell lung cancer: a meta-analysis. Cancer 1996 Jun 1;77(11):2413-2414.
- Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb non-small cell lung cancer. A meta-analysis. Cancer. 1995;76(4):593-601.
- Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 1996 Nov 1;125(9):723-729.
- Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2016 Mar 20;34(9):953-962.
- Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III Study of Cisplatin, Etoposide, and Concurrent Chest Radiation With or Without Consolidation Docetaxel in Patients With Inoperable Stage III Non-Small-Cell Lung Cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008 December 10, 2008;26(35):5755-5760.
- Vokes EE, Herndon JE,2nd, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007 May 1;25(13):1698-1704.
- Tsujino K, Kurata T, Yamamoto S, Kawaguchi T, Kubo A, Isa S, et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol 2013 Sep;8(9):1181-1189.
- De Ruysscher D, Vansteenkiste J, Belderbos J, Decaluwe H, Dingemans AM. The Optimal Local Treatment of Stage IIIA-N2 NSCLC: Is the Issue Finally Settled? J Thorac Oncol 2016 Mar;11(3):284-286.
- Bezjak A, Temin S, Franklin G, Giaccone G, Govindan R, Johnson ML, et al. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J Clin Oncol 2015 Jun 20;33(18):2100-2105.
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015 Feb;16(2):187-199.
- Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010 Feb;5(2):220-228.
- Broderick SR, Patel AP, Crabtree TD, Bell JM, Morgensztern D, Robinson CG, et al. Pneumonectomy for Clinical Stage IIIA Non-Small Cell Lung Cancer: The Effect of Neoadjuvant Therapy. Ann Thorac Surg 2016 Feb;101(2):451-7; discussion 457-8.
- Gilligan D, Nicolson M, Smith I, Groen H, Dalesio O, Goldstraw P, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007 Jun 9;369(9577):1929-1937.
- Albain KS, Swann RS, Rusch VW, Turrisi AT,3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009 Aug 1;374(9687):379-386.
- van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007 Mar 21;99(6):442-450.
- Eberhardt WE, Pottgen C, Gauler TC, Friedel G, Veit S, Heinrich V, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015 Dec 10;33(35):4194-4201.
- Cerfolio RJ, Bryant AS, Jones VL, Cerfolio RM. Pulmonary resection after concurrent chemotherapy and high dose (60Gy) radiation for non-small cell lung cancer is safe and may provide increased survival. Eur J Cardiothorac Surg 2009 Apr;35(4):718-23; discussion 723.
- Shan WW, Sun S, Laskin JJ, Ho C, Melosky BL, Carolan H, et al. Favorable outcomes with chemoradiation and surgery for locally advanced non-small cell lung cancer: The BC Cancer Agency Vancouver experience. ASCO Meeting Abstracts 2012 May 30;30(15_suppl):7020.
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006 Jun;131(6):1229-1235.
- Port JL, Kent MS, Korst RJ, Keresztes R, Levin MA, Altorki NK. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2004 Jan;77(1):254-9; discussion 259.