The correct answer is 4.
Rationale: Mosteller equation (round to 2 decimal places)
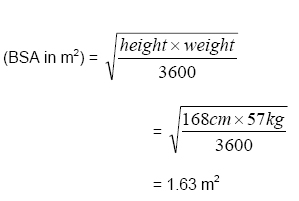
G.F’s Previous DOXOrubicin:
DOXOrubicin dose in BRAJAC protocol = 60 mg/m2/cycle x 4 cycles.
60 mg/m2 x 1.63 m2 = x 4 cycles = 391 mg.
Alternate calculation method: 60 mg/m2 x 4 = 240 mg/m2.
Lifetime Dose:
It is recommended that cardiac function be monitored when the cumulative dose of DOXOrubicin reaches 300 mg/m2, as outlined in the Clinical Pharmacy Guide Appendix B: Guidelines for Anthracycline Monitoring Thresholds, and in the LYCHOPR protocol Precautions section.
300 mg/m2 x 1.63 m2 = 489 mg.
Amount of DOXOrubicin G.F. can receive before exceeding the recommended maximum lifetime dose monitoring threshold:
Recommended maximum – dose already received = allowable amount remaining
489 mg – 391 mg = 98 mg
Alternate calculation method: 300 mg/m2 – 240 mg/m2 = 60 mg/m2.
Number of Cycles of LYCHOPR before recommended maximum is reached:
DOXOrubicin dose in LYCHOPR protocol dose is
50 mg/m2/cycle
50 mg/m2 x 1.63 m2 = 81 mg.
The maximum number of cycles before the recommended maximum dose is exceeded is 1 cycle.
Allowable amount remaining ÷ dose per cycle
98 mg ÷ 81mg = 1.2 cycles
Alternate method for calculation:
60 mg/m2 ÷ 50 mg/m2 = 1.2 cycles
Further therapy with DOXOrubicin after the first cycle of LYCHOPR should require that cardiac assessment be performed first, as detailed in the Precautions Section of the LYCHOPR protocol.
